“Something Bigger Than Me”: Launching Sanofi’s Biosafety Lab to Fight COVID-19
Early in 2020, before COVID-19 turned the world upside down, biologist Jennifer Umstead was gearing up for a new role at Sanofi’s Swiftwater, Pennsylvania campus in laboratory operations, biosafety and surveillance for Sanofi’s vaccine development program.
Initially Jennifer was charged with managing operational support of a new high containment facility for poliovirus research. As a parent of twins born prematurely at 27 weeks with severe heart and lung complications, Jennifer was drawn to working in an area of infectious disease that primarily impacts children.
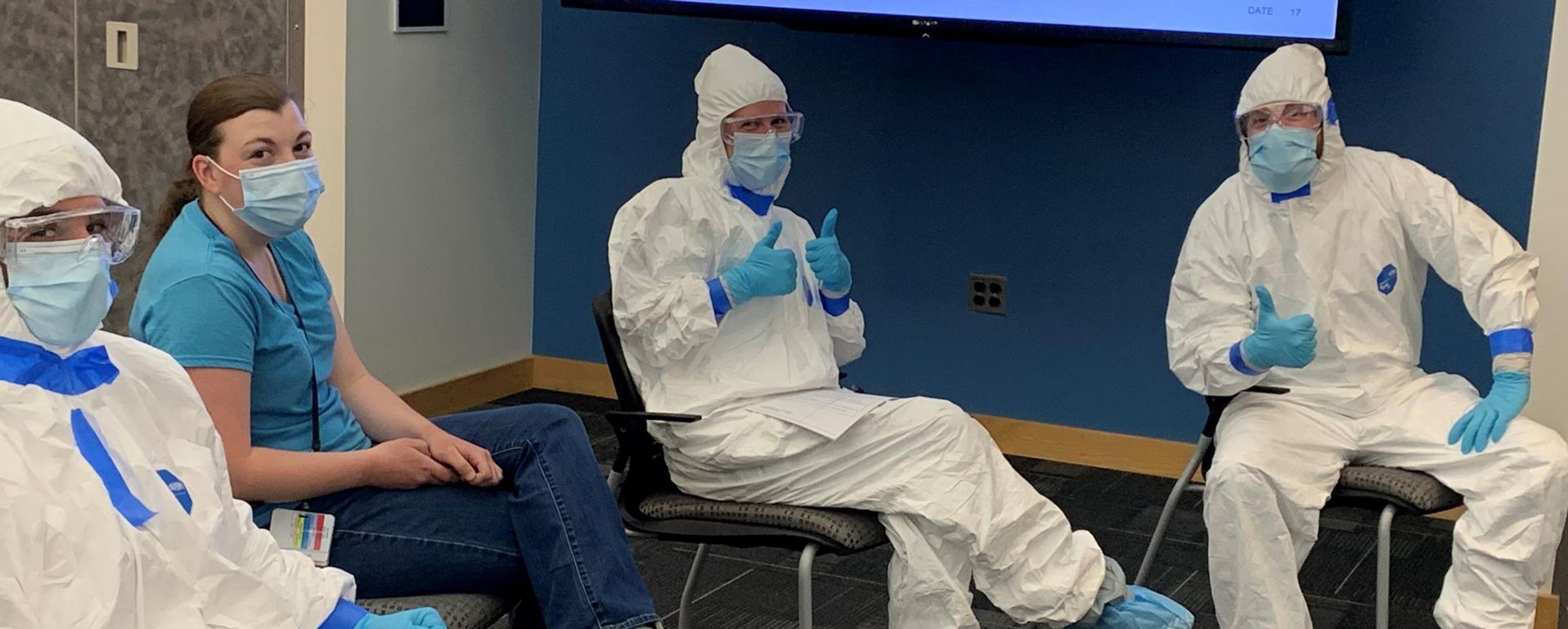
She thought she had months to get the lab ready when COVID-19 burst onto the scene and became an urgent global health threat. Rather than ease into her new role, Jennifer spent her first day on site in an 8-hour biosafety level 3 (BSL-3) training course to get the lab mobilized and fully operational for the novel virus – including containment of live virus – and she’s only now beginning to exhale.
“When I applied for this position, COVID-19 was not a factor. Originally the high containment facility was primarily designed to facilitate the global eradication of poliovirus and comply with CDC and WHO GAPIII guidelines,” notes Jennifer. “The facility was nearing completion when I accepted the position, but we were nowhere ready for go-live. We had it up and running within weeks.”
Poliovirus will resume being a focus of the BSL-3 high containment facility Jennifer manages, but for now COVID-19 vaccine research is the priority. Biosafety level 3 labs contain, handle and test lethal infectious agents like coronavirus with known potential for respiratory transmission.

Analysts typically spend two to three full days in the BSL-3 facility performing their work under intense conditions. Handling biological material and live virus samples in the lab requires rigorous safety protocols. Biosafety hazard suits (affectionately known in the BSL-3 as the big white bunny suit), safety glasses, respirators and double layers of gloves and shoe covers are needed just to enter the lab. Analysts do not leave the lab once inside. There are no bathroom or food breaks, and the work requires physical stamina and a clear head to follow the stringent safety protocols inside the lab.

“We tell them if they’re tired or they don’t feel well, don’t go into containment,” Jennifer explains. “Every single thing they do has to be risk assessed and thought out even if they’ve done it 10 times before.”
The biosafety operations team learned quickly to adapt and streamline safety and surveillance practices during the pandemic.
“Every group looked at their process and found new ways of doing things. We had a lot of support from Sanofi, which was critical, as well as from one another,” Jennifer notes. “Our team made every effort to support one another. Each day brought a new challenge but if you’re keeping an open mind and staying positive, you can work through and overcome any obstacle.”
Challenges intensified for Jennifer at home too. Just a few months after the pandemic hit the U.S., Jennifer’s daughter Rylee, then three years old, was scheduled for heart surgery to repair a defect to her aorta that causes fluid to build up in the lungs. As micro preemies, Rylee and her twin brother Ryder are especially vulnerable to COVID. In addition, her father-in-law, who frequently helps out with the kids, was undergoing treatment for prostate cancer.
“To say I had some anxiety about bringing COVID home to my children and my father-in-law is definitely an understatement,” says Jennifer. “I was certainly hesitant at first about this new role. But having spent years in hospitals and doctor’s offices, I would never jeopardize the health of fragile loved ones and my kids for my own career advancement. After careful consideration, I knew I was the right person for the job.”
On tough days, Jennifer focuses on the big picture.
“My role is to support the analysts while they work in extremely tough conditions so that no one dies from a vaccine-preventable disease,” she says. “Being part of something bigger than me is rewarding and I think everyone at Sanofi plays a part in the process. Once COVID hit, I had an even more personal vested interest in our current BSL-3 project due to having children at home who would have suffered greatly had they been exposed. I have an especially high interest in making the world a safer place, and in the end, we all want our family and our friends to be safe and live healthy and happy lives. COVID made that much more apparent.”

Jennifer Umstead stands outside the BSL3 lab.