Why Helping to Protect Infants Against Serious RSV Lung Infection is Personal to Me

By Kristine Santella, Learning and Development Manager – Commercial Operations
In early November of 2019, when my daughter Emilia was 9 months old, she came down with minor cold symptoms just a few weeks after starting daycare. These symptoms progressed quickly, and within days, she was admitted to the Pediatric Intensive Care Unit. Once there, we learned that she had bronchiolitis due to respiratory syncytial virus (RSV). We learned in real time just how unpredictable RSV can be – it can go from cold-like symptoms to hospitalization in less than a week. And while severe RSV is rare, it is the leading cause of hospitalization in babies under age 1.
Emilia received supportive care, until she fought through the virus on her own. She is now a healthy five-year old, but the fear and helplessness we felt during the experience has always stayed with me and my husband.
While sharing Emilia’s story on one occasion last year, I stated that if we were ever fortunate enough to have more children, being able to help protect them from a severe RSV lung infection like Emilia’s would be amazing. What I didn’t know at that time was that we were already expecting not one, but two more children. I was pregnant with twins due in November, right at the start of the RSV season (typically fall-spring, but can vary by local area).
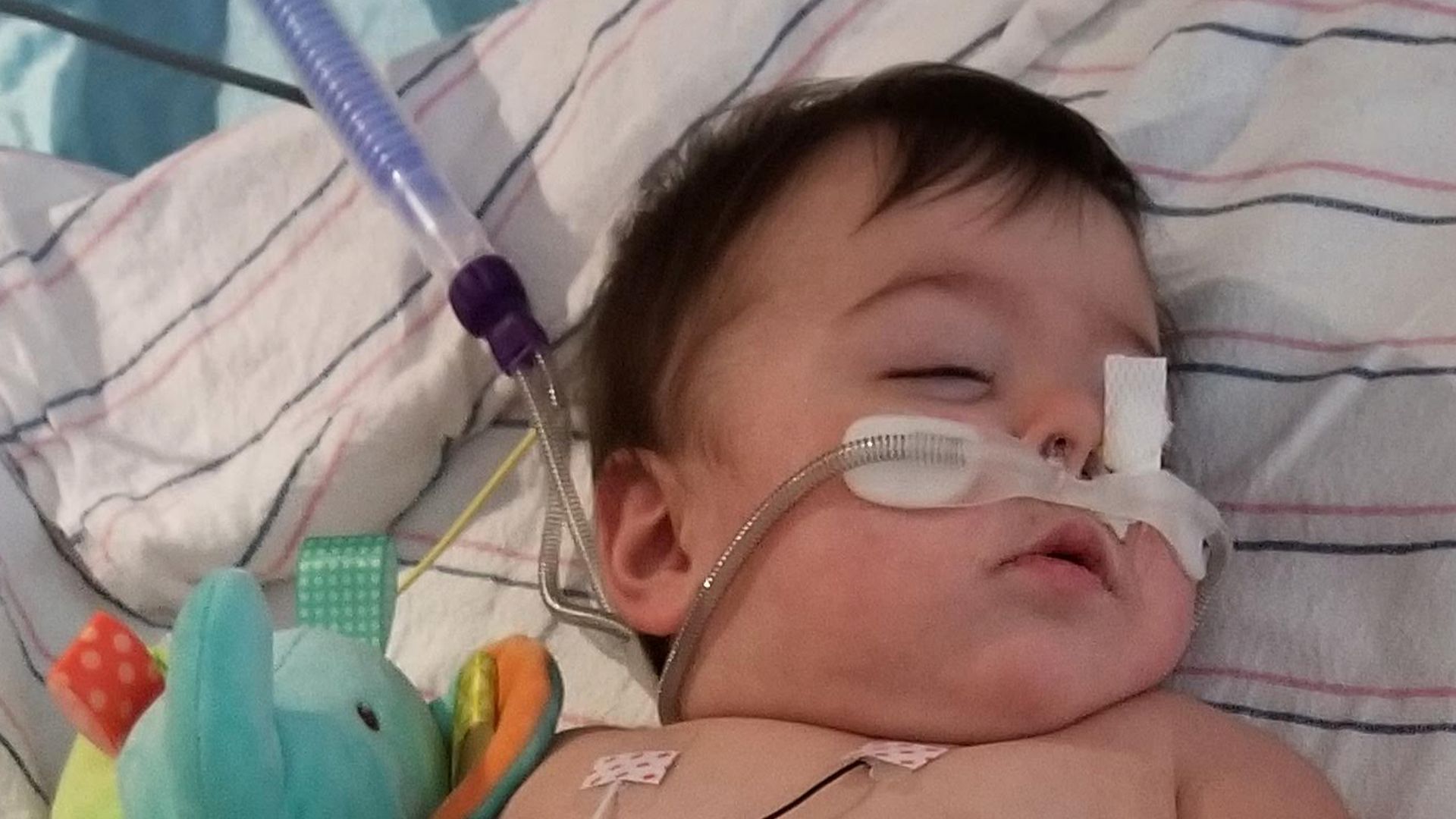
Emilia when she was hospitalized with RSV in 2019.
Throughout my pregnancy, I happened to work on a comprehensive training program for Sanofi’s sales team on RSV and Beyfortus® (nirsevimab-alip) 50mg and 100mg injection. Beyfortus is a prescription medicine used to help prevent a serious lung disease caused by respiratory syncytial virus (RSV) in:
- Newborns and babies under 1 year of age born during or entering their first RSV season.
- Children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season.
Beyfortus should not be given to children with a history of serious allergic reactions to nirsevimab-alip or any of the ingredients in Beyfortus.
Beyfortus may not protect all children. See below for additional Important Safety Information.
It was exciting to be involved in this effort after what we had experienced with Emilia, and I hoped that Beyfortus would be available for the twins by the time they were born. I was well-informed regarding the efficacy and safety of Beyfortus, the results of the clinical trials, and potential side effects, so it was the right decision for me and my husband to have our babies immunized.
My twins, Nathan and Nolan, were born at 38 weeks in early November 2023. They were beautiful, healthy babies, weighing in at 7 lbs 4 oz and 5 lbs 14 oz, respectively. They had no complications, and we were discharged from the hospital three days after their birth. The day after we left, Nathan and Nolan had their first visit with their pediatrician. After we inquired about Beyfortus, the pediatrician administered a 50 mg dose to each baby. At our next office visit, the pediatrician asked if the boys had experienced any side effects, which they had not.

Nathan and Nolan at 3 weeks old.
Nathan and Nolan have now experienced their first RSV season. Emilia continued to attend preschool, and we certainly had our fair share of colds throughout the season – but the boys never had more than a little cough or congestion. Without Beyfortus, this RSV season could have been a frightening time for us. The fear of both our babies being hospitalized was never far from our minds. But we knew that the boys had an extra layer of protection against a serious lung infection, like pneumonia caused by RSV. While there are many anxieties that come with having newborn twins, for us, the fear of RSV was much lower on the list.
IMPORTANT SAFETY INFORMATION
Your child should not take Beyfortus if your child has a history of serious allergic reactions to nirsevimab-alip or any of the ingredients in Beyfortus.
Before your child receives Beyfortus, tell your healthcare provider about all of your child’s medical conditions, including if your child:
- has ever had a reaction to Beyfortus.
- has bleeding or bruising problems. If your child has a problem with bleeding or bruises easily, an injection could cause a problem.
Tell your healthcare provider about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your infant should not receive a medicine called palivizumab if they have already received Beyfortus in the same RSV season.
Serious allergic reactions have happened with Beyfortus. Get medical help right away if your child has any of the following signs or symptoms of a serious allergic reaction:
- swelling of the face, mouth, or tongue
- difficulty swallowing or breathing
- unresponsiveness
- bluish color of skin, lips, or under fingernails
- muscle weakness
- severe rash, hives, or itching
The most common side effects of Beyfortus include rash and pain, swelling, or hardness at the site of your child’s injection. These are not all the possible side effects of Beyfortus. Call your healthcare provider if you have questions about side effects.
INDICATION
Beyfortus is a prescription medicine used to help prevent a serious lung disease caused by Respiratory Syncytial Virus (RSV) in:
- Newborns and babies under 1 year of age born during or entering their first RSV season.
- Children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season.
- Please see full Prescribing Information, including Patient Information, for more details.
MAT-US-2405475-v1.0-06/2024